Buy Online
Protectyn®
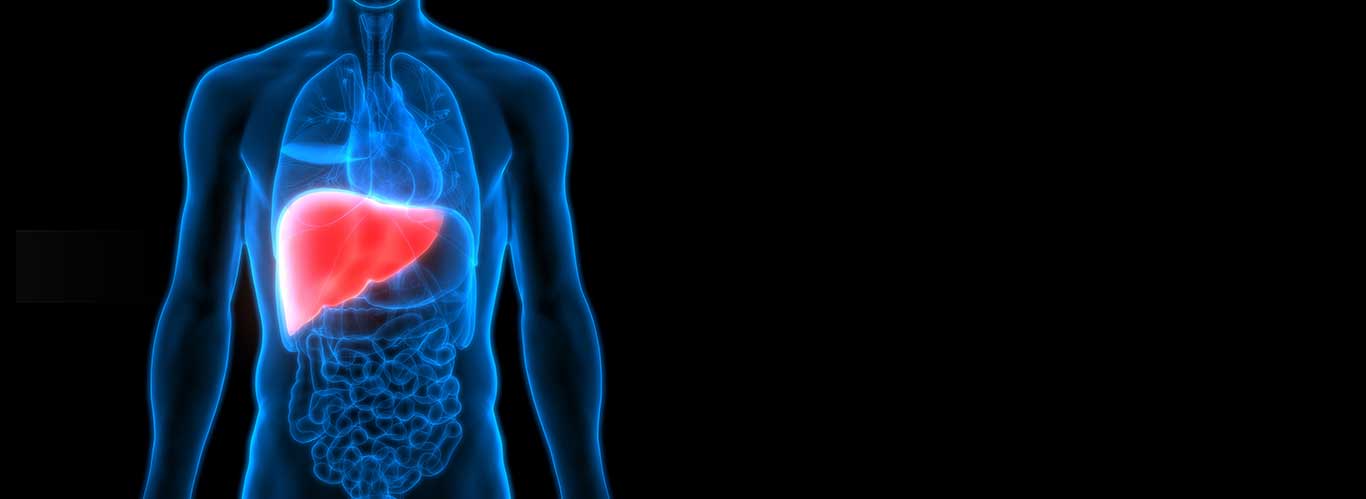
Protectyn® is a very special form of colostrum that is naturally boosted with antibodies and other components to protect infants from diseases of the digestive system....

Travelan®
Travelan® is an over-the-counter oral polyclonal antibody therapy that helps reduce the risk of Travellers’ Diarrhoea when you are travelling overseas....
Clinical Insights & Updates
Recent Articles
Immuron marks surge in Travelan® sales on eve of entry into US via Walmart.com
10 April 2024
Immuron Ltd (NASDAQ:IMRN, ASX:IMC), a biopharmaceutical company headquartered in Australia, says sal...
read moreGuarding Gut Health on the Go: The Travelan® Advantage
10 April 2024
The Not Old Better Show, Art of Living Interview Series. Today's episode takes us on a fascinati...
read moreClinical data imminent!
6 February 2024
Immuron CEO Steve Lydeamore speaks about the clinical work it is doing with IMM-124e and IMM-529, it...
read moreBeating bacteria just got boosted
11 November 2023
We might like experiencing new places, but our tummies don’t always agree with us. Here’s somethi...
read more